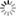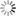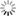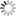|
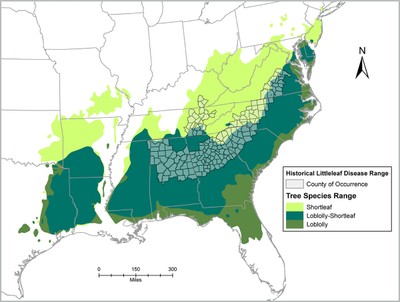
Southern Regional Extension Forestry Littleleaf disease is a forest health concern for several pine species in the southeastern United States. This disease is a particular problem for shortleaf pine (Pinus echinata Mill.) and is one reason why this species is less widely planted compared with other pines. Littleleaf disease results from a combination of biological factors and site characteristics, when combined with a susceptible host tree. Some factors are more important - i.e., they have a primary role in disease formation - while others have a secondary role, and mostly contribute to making disease symptoms worse. Littleleaf disease contributed to the decline of shortleaf pine as an important commercial species in the Piedmont region. Following major losses of shortleaf pine during the 20th century, the species was frequently thinned out of stands and rarely replanted. Loblolly pine's (Pinus taeda L.) faster growth rate and lower littleleaf disease susceptibility was more favorable for replanting on Piedmont sites compared to shortleaf pine. Infrequent reports of littleleaf disease in the Piedmont region today may be a direct result of shortleaf pine's less common occurrence. Additionally, since infected trees are more susceptible to insect outbreaks (e.g. southern pine beetle), littleleaf disease may be misdiagnosed as an insect issue, or undiagnosed altogether. This fact sheet outlines the history and causes of littleleaf disease, the symptoms used for identification, and management recommendations. HistoryLittleleaf disease was first documented in Alabama in 1934, and by the 1950s, it was reported in scattered stands over 30 million acres (Fig. 1). The name “littleleaf disease” was coined because stunted needles, or “little leaves”, are an early visible symptom. Historically, littleleaf disease occurred on poorly drained and heavily eroded sites in the Piedmont region, an area rich in agricultural history. Farming on typical Piedmont sites- low hills, narrow valleys and clay-textured soilsoften led to severe erosion, altered soil structure, and compaction in the absence of modern soil conservation practices. As a result, much of the fertile, well-drained topsoil eroded leaving less fertile, more poorly drained subsoil as a rooting medium for regenerating pines. The following excerpt from Bratislav Zak's littleleaf research in the 1960s describes how land use change exacerbated the disease in southern pines:9 "Originally, the deep, friable, rich soils of the Piedmont bore climax forests of oak, hickory, and other hardwoods, with scattered pine. Although many sites were underlain with heavy and poorly drained subsoils, tree growth was good when deep and rich surface soils were present. As these stands were removed and the soils cultivated and planted to row crops, sheet erosion began and continued. Rather generally, when row-crop fields are abandoned in the Piedmont, the land seeds in naturally to shortleaf and loblolly pines. This has happened on millions of acres of former cottonfields. On many such fields the trees have later been harvested, and the area farmed and abandoned again. Then the wind sows pine seed once more. Each succeeding cycle has resulted in decreased production as topsoil washed away to redden Piedmont rivers. Surface horizons originally 18 inches and more in depth now measure only a few inches, and often bare subsoil is exposed. As the soil mantle shrinks, forcing root growth of trees closer to and finally into the subsoil, growth declines. The effect is less severe where the subsoil is friable, light, and adequately drained. On plastic, heavy, and poorly drained subsoils, however, growth retardation is marked and continuing. It is on such sites that littleleaf is found." In the early to mid-20th century, shortleaf and loblolly pine were the primary pine species re-colonizing abandoned agricultural fields, and littleleaf disease led to major financial losses in both.1 Approximately 35% of the commercial shortleaf pine acreage east of the Mississippi River was reported to be affected by littleleaf disease, with the highest occurrence reported in Alabama, Georgia, and South Carolina. Table 1. Factors influencing littleaf disease in the Piedmont region of the southeastern U.S.
CauseThe primary pathogen associated with littleleaf disease is the soil-borne water mold (a fungus-like organism), Phytophthora cinnamomi Rands. It is a leading cause of "die-back" and mortality in a variety of agricultural crops and native plants worldwide. In the U.S., many commercial and ornamental trees, shrubs, and horticultural plants are highly susceptible to P. cinnamomi. Phytophthora cinnamomi can remain dormant in the soil or infected roots for up to six years. In warm summer temperatures and water-saturated soils, especially when soil remains saturated for more than 8 hours,3 P. cinnamomi breaks dormancy and becomes active. The spores are moved through the soil in water (through rainfall, irrigation, etc.) and when they come in contact with a susceptible tree's fine root system, they can infect the tree through the root tips. Infection damages the root tips, leading to decreased root growth and nutrient and water uptake-all limiting factors to tree growth and health. Healthy trees are more resistant to pests and diseases than trees under stress. As such, any factor that stresses a tree will contribute to decreasing tree health and increase the risk of pest or disease issues. Certain site conditions that cause tree stress also favor the development of littleleaf disease (Table 1). These include poor soil drainage or soil that is subject to moisture fluctuations, inadequate aeration, severe erosion, and nutrient deficiency.1 Though these sites may be suitable for some tree species, they are generally not ideal for southern pines. Phytophthora cinnamomi is common throughout the Piedmont region of the southeastern U.S. Due to its presence, careful site selection
and preparation are critical when regenerating highly susceptible pine species in this region, such as shortleaf pine. Understanding site factors that increase the likelihood of littleleaf disease help forest managers make better management decisions. The two most important site factors found to influence littleleaf disease are soil erosion and internal drainage.1 Fortunately, these characteristics can be evaluated relatively easily in the field and integrated into a site management plan. Soil erosion is the loss of soil though wind or water and is measured by the amount of topsoil (also known as the A horizon) that remains. In the Piedmont region, most of the original topsoil was better drained, aerated, and contained more nutrients than the clay subsoil underneath. The amount of currently remaining topsoil provides a good indication of the quality rooting depth for pine trees: the greater the topsoil depth, the better. If topsoil is minimal, but the clay subsoil contains a well-drained, sandy textural component, it may provide a suitable growing medium with site prep techniques and fertilizer amendments. On the other hand, if topsoil is minimal and the clay subsoil is compacted or poorly drained, site prep techniques such as subsoiling will be necessary to ameliorate drainage problems. Soil internal drainage describes the rate at which water moves downward through soil and can indicate how long soils remain saturated with water following precipitation. Permeability (the ability of water and air to move through the soil), consistency (the strength at which soil is held together), and depth to mottling (how deep in the soil profile before you encounter spots of color, either grey or brown, that indicate waterlogging or water table depth) were found1 to be the best ways to measure a soil’s internal drainage in the field. (See Management Recommendations: Risk Assessment A for information on measuring these soil characteristics). Soil internal drainage is an important measure of a site’s littleleaf disease risk since P. cinnamomi infection rates increase when the soil is saturated more than 8 hours.3 In the Piedmont region, approximately 90% of pine tree fine roots are contained in the upper 12 inches of soil. For this reason, ensuring that good internal drainage extends deeper than the top 12 inches of soil (36 inches or more is recommended) may help reduce infection rates. If, upon site evaluation, the site contains inadequate soil internal drainage, site prep techniques such as subsoiling will be necessary to improve site drainage. On sites with good internal drainage, healthy, vigorous trees can usually outgrow root damage caused by the pathogen.6,9 On sites that are eroded and have poor drainage, other factors may further increase tree susceptibility to littleleaf disease. For instance, reduced concentrations of surface soil nitrogen and minimal soil organic matter may lead to a lack of resources for the tree to replace roots lost to P. cinnamomi. Sites with a low site index, slopes greater than 10%, and more northern or eastern facing aspects were also found1 to have a higher risk of the disease. Several species of soil fungi (Pythium spp.) and plant parasitic nematodes can also add to decline from littleleaf disease-affected trees. Pine SusceptibilitySeveral southern pine species have demonstrated varying degrees of susceptibility to littleleaf disease. Shortleaf pine is highly susceptible, while loblolly pine is considered moderately susceptible.6 Longleaf, slash, pitch, and Virginia pine have shown littleleaf disease symptoms and damage,10 but their susceptibility is generally considered low. Shortleaf pine’s higher susceptibility is problematic because the age when symptoms first appear (20-30 years) coincides with and may be earlier than the species’ typical harvest age (> 40 years). Symptoms appear later in less susceptible trees such as loblolly pine. Further, loblolly pine is commonly grown on shorter rotations (25-35 years) than shortleaf pine and is usually harvested before littleleaf disease symptoms impact productivity. Despite differences in silviculture and management among the southern pines, shortleaf pine has a higher susceptibility to littleleaf disease, even on “good” sites, and shortleaf pine mortality from littleleaf disease has exceeded other southeastern pines.2 SymptomsThe first visible symptom of littleleaf disease is stunted, yellow or chlorotic needles (Table 2, Fig. 2). After 1-2 years, additional symptoms occur, Table 2. Visible symptoms of littleaf disease.
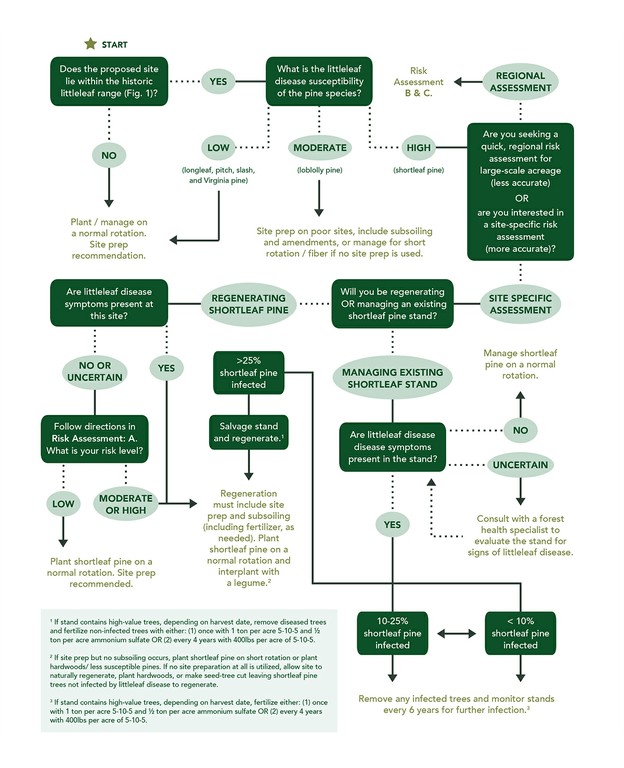
such as: reduced twig length, retention of current year needles, a concentration of needles at the end of twigs, a thinning crown, abundant and persistent cone crops with or without viable seeds, small cones, and sprouting at the base of the stem (Fig. 3). Trees can die within 1 year on poor sites, or survive as long as 12-15 years on better sites; however, on average, trees die within 6 years after symptoms appear.4 Prior to these visible symptoms, another, less obvious indication of littleleaf disease is fine root damage, which can be seen as root lesions or root mortality. Because fine roots are responsible for nutrient and water uptake, their damage leads to aboveground symptoms. For example, chlorotic needles and stunted twig length occur because the roots are unable to obtain enough nutrients for the tree. Root damage can lead to thinning crowns, which can lead to reduced photosynthesis and diminished radial growth. Sometimes, radial growth reduction, which can be measured with an increment borer, can occur 10-15 years prior to the appearance of visible symptoms. Management RecommendationsPhytophthora cinnamomi is impossible to eradicate at the stand level and development of natural resistance to the disease through breeding and genetic research takes significant time and resources, therefore, careful site selection or improvement of problematic site and soil conditions is important. The use of P. cinnamomi-free nursery or seedling stock may also help decrease disease incidence, if available. Consulting with a state forest health professional, forest manager, certified soil scientist, or consulting forester is highly recommended when prescribing management plans in areas historically impacted by littleleaf disease. See the Resource Information section at the end of this document to help you find a local forestry professional. The following diagram (Fig. 4) can be used to asses a site/ region’s littleleaf disease risk and, based on the risk level, guide management decisions of southern pines.1,6,7,8 The diagram is followed by descriptions of three risk assessment methods (A, B, and C), all referenced in the diagram. Risk Assessment MethodsThree methods were developed to assist forest managers and other natural resource professionals in assessing site-specific or regional littleleaf disease risk. The Site Hazard Rating (Risk Assessment A) is the most effective way to rate the littleleaf disease risk of a specific site/stand but requires field evaluation and local soils knowledge. Two regional evaluation methods, The Shortleaf Pine Initiative’s Site Suitability & Decision Support Tool and the Soils Series Risk Rating, were developed to assess risk across stands or over large-scale acreage (Risk Assessment B and C). They do Figure 4. Littleleaf disease risk assessment and management recommendations decision support diagram.*
Visit www.shortleafpine.com and www.southernforesthealth.net for more information. *This diagram was developed based on research by: Campbell and Copeland (1954, USDA, Circ. 940, 41 p.), Mistretta (1984, USDA Forest Service, Insect and Disease Leaflet No. 20), Oak and Tainter (1988, USDA Forest Service- Southern Region, Protection Rep. R8-PR 12, 14p.), and Roth et al. (1948, Journal of Forestry, 46, 578-587 p.). not require site evaluation to use and are based on Natural Resource Conservation Service (NRCS) county-level soils maps. Typically, the smallest-scale NRCS soil survey is one soil sample per 40 acres. As such, this scale may not account for local variations in soils and may not be appropriate for small acreages. An onsite evaluation (Risk Assessment A) should eventually accompany regional evaluation methods as more site-specific management plans are developed. A) Site Hazard Rating (Table 3)Though this risk assessment method was developed in the 1950s, when the greatest losses in southeastern pines were incurred by the disease, it is still regarded as the most effective way to assess littleleaf disease risk today.2 The Site Hazard Rating table is recommended for sites where shortleaf and loblolly pine are not currently growing and/ or littleleaf disease is not present.6 The method rates a site’s risk based on field observations of two soil characteristics known to influence littleleaf disease, soil erosion and internal drainage.1 Because soils vary greatly over even a small area, a field-based risk assessment is more reliable for assessing a site’s littleleaf disease risk than regional risk assessments based soil series or mapping units (Risk Assessments B & C), which don’t account for local variations in soil. For this reason, if regenerating highly susceptible species (shortleaf pine) in the historic littleleaf disease range, the Site Hazard Rating should be utilized during site evaluation. The method is easy to use, requiring simple tools, but requires knowledge of soil evaluation and description. If needed, consult with a soil scientist or natural resource professional (with soils experience) in your area to assist with the Site Hazard Rating. Refer to the Resource Information section at the end of this document to locate a soil scientist in your area. Table 3. System for rating field sites for littleleaf disease hazard.1
*This field rating system should be used by a forestry professional or someone with knowledge of local soils. select the site to be evaluated and then decide how you will examine the soil. The soil can be evaluated by either (1) digging a soil pit to a 3-4 foot depth, ideally with one vertical wall, or (2) examining a small hole to a 3 foot depth using an auger, shovel, or post hole digger (a post hole digger is preferred because it minimizes soil mixing or disruption compared to the other tools). Using Table 3 as a guide, first evaluate the degree of soil erosion in your observational soil pit and select the best erosion rating. Next, evaluate internal drainage characteristics of the soil and select the best ratings for each of the three sections (consistence, permeability, and mottling). To measure subsoil consistence, the soil should be slightly moist (between air dry and field capacity) and originate from an undisturbed B horizon, if possible. To measure where the reduction in permeability begins (inches from the soil surface to a specified depth), measure to the depth where the following occurs: resistance to digging, few large pores or cracks, soil layer denseness, or soil layer texture.2 Permeability changes typically occur in the B horizon. And finally, to measure subsoil mottling, rate the quantity of brown and gray spotting within the soil background color, or soil matrix, of the B-horizon. Though only quantity is rated in this method, the mottling depth can provide some indication of where water saturation or impeded downward flow is occurring. After rating soil erosion and internal drainage characteristics, total points from all sections to rate the hazard of your site. A total of 0 to 50 points indicates a high littleleaf disease hazard site whereas a low hazard site will range from 75-100 points. The rating is used to guide further management recommendations made in this fact sheet. B) The Shortleaf Pine Initiative’s Site Suitability & Decision Support ToolThis online resource (Fig. 5) contains a spatial (map) layer designed to regionally rate a site’s soil limitation for shortleaf pine regeneration or management. This layer incorporates soil and site factors known to influence littleleaf disease susceptibility,1 and can be used to broadly avoid areas where soils may lead to increased littleleaf susceptibility. The spatial layer is based on NRCS county-level soils data across the shortleaf pine native range (Fig. 1) and was modeled on soil factors known to influence littleleaf disease. After navigating to the website, select the appropriate mapping search options (state, county, or latitude-longitude). Click on Data Layers and check boxes for Historic Littleleaf Counties and Shortleaf Pine Soil Limitation. Next, click on Legend. You can zoom to the desired location using the legend to provide soil limitation rating. These levels roughly equate to littleleaf susceptibility. For example, not limited is low littleleaf susceptibility, and very limited equates to high littleleaf susceptibility. Click on the i beside the data layer, Shortleaf Pine Soil Limitation, for more information on how this spatial layer was created. The Decision Support Tool also provides NRCS site index values for shortleaf pine. Select the spatial data layer, Shortleaf Pine Site Indices, and click on the Legend tab to obtain a site index value for your site. C) Soil Series Risk Rating (Table 4)A regional risk assessment for littleleaf disease was developed based on soil series.7 Soil series are defined as having similar color, texture, structure, mineral and chemical composition, and consistence and are found to behave similarly to agricultural, residential, and forestry uses. Several soil series in the Piedmont region were evaluated based on data used to develop the above mentioned Site Hazard Rating. Associations of soil erosion and internal drainage with soil series were made and a rating was developed. This method is recommended as a non-field based Table 4. Littleleaf disease risk level by Natural Resource Conservation Service (NRCS) soil series.7
method to assess stands or for large-scale forest planning purposes. To use this method, navigate to the NRCS Web Soil Survey (http://websoilsurvey.sc.egov.usda.gov/) to identify soil series on the proposed forest restoration or management site and assess the risk level according to Table 4. Conclusionsorganism (specifically, a water mold), infects the fine roots of susceptible southern pine trees. Symptoms of the disease can be misdiagnosed as nutritional deficiencies or may appear as the after effects of bark beetle attack. On average, trees die six years after the first symptoms appear but mortality can occur within a year on poor sites. Susceptible trees are most at risk on sites within the historic littleleaf range, and especially when soils are severely eroded and have poor internal drainage. Other risk factors include low soil nitrogen content, which aids in root growth, and the presence of the fungal Pythium species, which can damage fine roots. Due to littleleaf disease and several other factors, shortleaf pine abundance has decreased by 53% since the 1980s. Shortleaf pine restoration efforts are currently underway across the species’ native 22-state range, which includes the Piedmont region of the southeastern U.S. and the historic littleleaf disease range. Though additional research is needed to advance restoration of shortleaf pine in areas susceptible to littleleaf disease, previous research efforts have established clear management guidelines. These guidelines recommend site selection, preparation, and amendments useful for reducing or managing risk of littleleaf disease.
AuthorsJ. Holly Campbell, Extension Associate, Southern Regional Extension Forestry and D.B. Warnell School of Forestry and Natural Resources, University of Georgia David R. Coyle, Forest Health Extension Associate, Southern Regional Extension Forestry and D.B. Warnell School of Forestry and Natural Resources, University of Georgia
SREF-FH-007 | SREF-SLP-019 | www.sref.info
Southern Regional Extension Forestry (SREF) is a diverse team of trained natural resource educators, IT specialists, graphic designers, communications and marketing experts, and media and content producers. SREF works closely with the Southern Land Grant University System, US Forest Service, and state forestry agencies to develop content, tools and support for the forestry and natural resource community. To find out more about SREF programs please visit www.sref.info. AcknowledgementsWe thank Chris Asaro, Becky Barlow, Michelle Cram, Bill Hubbard, Jason Jennings, Larry Morris, Brent Peterson, Andy Scott, Rob Sutter, Rob Trickel, and an anonymous reviewer for comments on a previous version of this document. References1Campbell, W.A., and Copeland, O.L. 1954. Littleleaf disease of shortleaf and loblolly pine. Circ. 940. USDA. 41 p. 2Campbell, W.A., Copeland, O.L., and Hepting, G.H. 1953. Managing shortleaf pine in littleleaf disease areas. Station Paper No. 25. Asheville, NC; USDA Forest Service, Southern Forest Experiment Station. 3Hwang, S.C., Ko, W.H., and Aragaki, M. 1975. A simplified method for sporangial production by Phytophthora cinnamomi. Mycologia 67: 1233-1234. 4Lawson, E.R. 1990. Shortleaf pine. Silvics of North America: Volume 1, Conifers. Ed. R.M. Burns and B.H. Honkala. Washington: U.S. Government Printing Office. P. 316-326. 5Lorio, P.L., Jr. 1966. Phytophthora cinnamomi and Pythium species associated with loblolly pine decline in Louisiana. Plant Disease Reports 50: 596-597. 6Mistretta, P.A. 1984. Littleleaf disease. USDA Forest Service. Insect and Disease Leaflet No. 20. Online: http://www.na.fs.fed.us/spfo/pubs/fidls/littleleaf/fidl-ll.htm 7Oak, S.W., and Tainter, F.H. 1988. How to identify and control littleleaf disease. Protection Rep. R8-PR 12. Atlanta GA; USDA. Forest Service, Southern Region. 14p. 8Roth, E.R., Toole, E.R., and Hepting, G.H. 1948. Nutritional aspects of the littleleaf disease of pine. Journal of Forestry 46: 578-587. 9Zak, B. 1961. Aeration and other soil factors affecting southern pines as related to littleleaf disease. Southeastern Forest Experiment Station, Forest Service Technical Bulletin No. 1248 USDA Forest Service. 10Zak, B. and Campbell, W.A. 1958. Susceptibility of southern pines and other species to the littleleaf pathogen in liquid culture. Forest Science 4: 156-161. ResourcesFor the location and phone numbers of state agencies in the southeastern U.S. providing forestry assistance and information, see the following websites: Alabama Forestry Commission: http://www.forestry.alabama.gov/ Arkansas Forestry Commission: http://forestry.arkansas.gov/Pages/default.aspx Florida Forest Service: http://www.floridaforestservice.com/ Georgia Forestry Commission: http://www.gatrees.org/ Kentucky Division of Forestry: http://forestry.ky.gov/Pages/default.aspx Louisiana Department of Agriculture and Forestry: http://www.ldaf.state.la.us/ Mississippi Forestry Commission: http://www.mfc.ms.gov/ North Carolina Forest Service: http://www.ncforestservice.gov/ Oklahoma Forestry Services: http://www.forestry.ok.gov/ South Carolina Forestry Commission: http://www.state.sc.us/forest/ Tennessee Division of Forestry: https://www.tn.gov/agriculture/section/forests Texas A&M Forest Service: http://texasforestservice.tamu.edu/ Virginia Department of Forestry: http://www.dof.virginia.gov/ For the location and phone numbers of University Extension personnel in the southeastern U.S. providing forestry assistance and information, see the following websites: Alabama Cooperative Extension System: http://www.aces.edu/main/ University of Arkansas Cooperative Extension Service: http://www.uaex.edu/ University of Florida’s Institute of Food and Agricultural Sciences (UF/IFAS): http://solutionsforyourlife.ufl.edu/ University of Georgia Extension: http://extension.uga.edu/ Kentucky Cooperative Extension Service: https://extension.ca.uky.edu/ Louisiana Cooperative Extension Service: http://www.lsuagcenter.com/ Mississippi State University Extension Service: http://extension.msstate.edu/ North Carolina Cooperative Extension: https://www.ces.ncsu.edu/ Oklahoma Cooperative Extension Service: http://www.oces.okstate.edu/ Clemson Cooperative Extension (South Carolina): http://www.clemson.edu/extension/ University of Tennessee Extension: https://extension.tennessee.edu/ Texas A&M AgriLife Extension: http://agrilifeextension.tamu.edu/ Virginia Cooperative Extension: http://www.ext.vt.edu/ To locate a soil scientist from the Natural Resources Conservation Service, locate your county from the following website map: Service Center Locator: https://offices.sc.egov.usda.gov/locator/app?agency=nrcs To locate a consulting forester: Association of Consulting Foresters: http://www.acf-foresters.org/acfweb. Click on "Find a Forester", then select your state in the "People Search – Public" search page. For more information on how to select a consulting forester, go to: http://www.uaex.edu/environment-nature/forestry/FSA-5019.pdf Figure Credits
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||